Bioreactors - function, application, and challenges
- Mira
- May 16
- 4 min read
What differences in available bioreactor types are critical for scaling bioprocesses

Bioreactors are essential tools in biotechnology, enabling controlled environments for cell culture, fermentation, and metabolite production. Their role is crucial in biopharmaceutical manufacturing, regenerative medicine, and large-scale cell expansion. As bioprocessing demands grow, bioreactor scale-up becomes a key challenge, ensuring that laboratory-developed processes translate effectively into industrial-scale production. Understanding different bioreactor types and their applications is critical for optimizing efficiency and product yield.
What is a bioreactor?
A bioreactor is a cultivation system designed to support and control biological reactions, providing optimal conditions for cell growth or biochemical transformations, mimicking in vivo conditions. These systems regulate factors like temperature, pH, oxygen levels, and agitation to ensure stable and reproducible results (1).
Bioreactors are commonly used for
Large-scale production of monoclonal antibodies, vaccines, and recombinant proteins (2)
Expansion of stem cells and generation of tissues for clinical applications such as regenerative medicine and cell therapy (1)
Fundamental research and process development in drug discovery, synthetic biology, and bioengineering (1)
Types of bioreactors
To meet the specific requirements of cell type, culture method, and production demands, various bioreactor designs exist. Each type serves unique process needs, balancing factors like scalability, shear sensitivity, and oxygen transfer.

Stirred-Tank Bioreactors
Stirred-tank bioreactors are among the most widely used systems for suspension cell cultures, particularly in large-scale biologics production. They utilize an integrated impeller system for continuous mixing, ensuring even distribution of nutrients. Spargers facilitate oxygenation by introducing gas into the media. However, the high shear stress generated by this system makes it unsuitable for sensitive cells (4).
Wave/Rocking Bioreactors
Wave/rocking bioreactors are an alternative with lower shear stress, making them ideal for shear-sensitive cells. These bioreactors use a disposable bag mounted on a rocking platform, creating a wave motion that gently mixes the culture. While this design minimizes contamination risk, it has limited volume capacity and may not provide optimal oxygen transfer for some cultures (3).
Fixed-Bed Bioreactors
Fixed-bed bioreactors are designed for adherent cell cultures, providing a stationary matrix for cells to attach while media flows through the system. These systems support high cell densities and efficient nutrient delivery. However, they are challenging to scale up and are susceptible to clogging (3).
Hollow Fiber Bioreactors
Hollow fiber bioreactors feature bundles of hollow fibers that allow controlled nutrient exchange while separating cells from waste. This system is beneficial for continuous culture applications, such as high-yield protein production and organ-on-chip models. While these bioreactors offer low media consumption and in vivo-like conditions, they express issues in oxygen transport and concentration gradients (3).
Single-Use Bioreactors
Single-use bioreactors employ disposable culture bags with integrated sensors and control systems, reducing contamination risks and simplifying operation. They are particularly useful in flexible manufacturing and process development. However, these systems are limited in their scalability, oxygen transfer and can lead to higher long-term costs and environmental concerns (5).
Scaling up bioreactors for cell culture
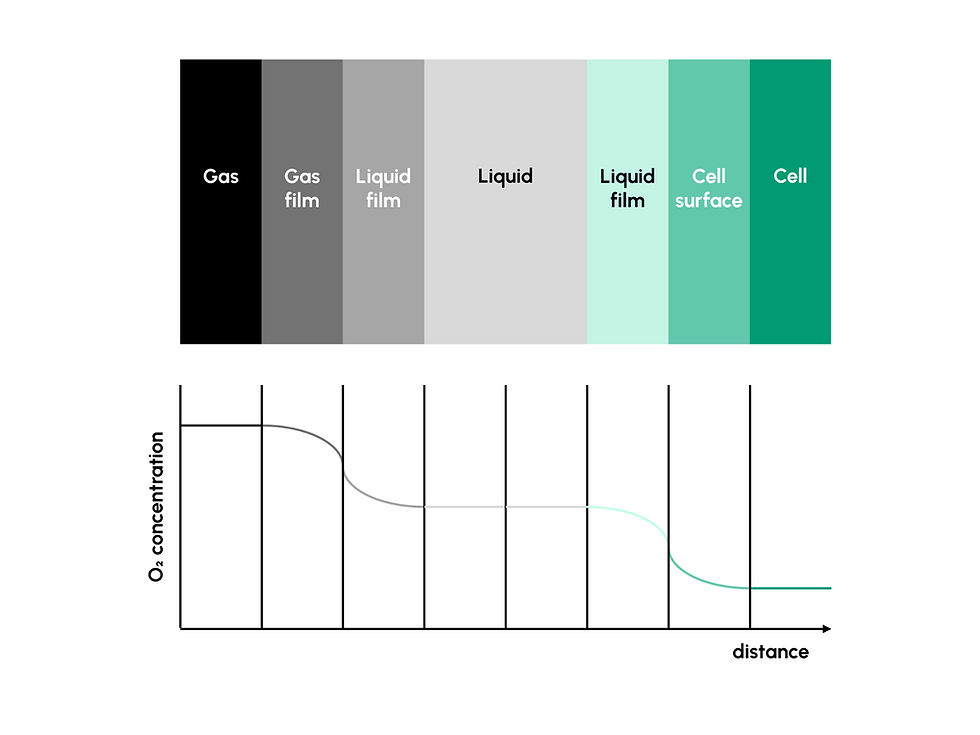
Transitioning from laboratory-scale to industrial-scale bioreactor processes requires careful optimization. Bioreactors that work for small-scale production with demanding cell lines might not be able to perform similarly on a larger scale. Bioreactor scale-up must therefore address the most critical factors of each cultivation: oxygen transfer limitations, nutrient distribution, shear forces, and process monitoring (3).
As the demand for biomanufacturing capacity grows, the need for efficient, cost-effective, and scalable cultivation solutions increases. Many traditional systems require trade-offs between efficiency, cost, and complexity, raising the question of novel bioreactor technologies that offer greater flexibility and scalability.
Bioreactors are indispensable in biotechnology and cell culture, enabling the controlled expansion of cells for diverse applications. Selecting the right bioreactor type and addressing scalability challenges are crucial for ensuring process efficiency and product quality. As technologies advance, innovations in bioreactors continue to enhance bioprocessing capabilities. By optimizing bioreactor systems, researchers and manufacturers can achieve more reliable, cost-effective, and scalable cell culture processes.
References
1. Kammoun A (2024) Bioreactors in Translational Medicine and Drug Development. J Curr Synth Syst Bio. 12:062.
2. Fang Z, Lyu J, Li J, Li C, Zhang Y, Guo Y, Wang Y, Zhang Y, Chen K. Application of bioreactor technology for cell culture-based viral vaccine production: Present status and future prospects. Front Bioeng Biotechnol. 2022 Aug 9;10:921755. doi: 10.3389/fbioe.2022.921755. PMID: 36017347; PMCID: PMC9395942.
3. Bellani CF, Ajeian J, Duffy L, Miotto M, Groenewegen L, Connon CJ. Scale-Up Technologies for the Manufacture of Adherent Cells. Frontiers in Nutrition [Internet]. 2020 Nov 4;7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7672005/
4. M.R. Spier, L.P. de Souza Vandenberghe, A.B. Pedroni 56 Medeiros et al. Applications of different types of bioreactors in bioprocesses, Bioreactors: Design, Properties and Applications (2011), ISBN: 978-1-62100-164-5
5. Salavadi SE, The Pros and Cons Of Single-Use Bioreactors, October 1 2010, Pharmeceutical Technology Europe available from https://www.pharmtech.com/view/pros-and-cons-single-use-bioreactorsr


Comments